Saturday, May 23, 2009
The "Essence" of Light
Introduction
Fluorescence, phosphorescence, bioluminescence; the “escence” of light. Through these different chemical phenomena, creatures, rocks, and clothing can emit light in a unique way. Fluorescence is the reason that your bowling ball glows in cosmic bowling (5). When you have a glow in the dark keychain, phosphorescence is at play. Fireflies thrive by using bioluminescence for mating (6). Light made by chemical reactions falls under the category of chemiluminescence. Studying the chemistry involved within these phenomena has solved many of these awe-inspiring and light-emitting mysteries.
Fluorescence and Phosphorescence
Phosphorescence and fluorescence are two processes that make objects glow. Although they are different, many similarities become apparent when further studied. They both appear mysterious because they emit more light than other objects next to them. Fluorescent molecules use their unique properties to harnesses energy that other molecules cannot (8). Phosphorescence continues to emit light after the source of energy is removed (5).
Fluorescence
In black light, some posters glow eerily. This cool effect is due to fluorescence (5). Fluorescence’s emission of light is due to the de-excitation of electrons. A form of energy such as thermal energy is applied. The electrons in the molecules become excited and move up one or more energy levels. The spin on the electron remains constant. This new electron is very unstable and quickly emits a photon to return to ground state. This photon is often at a wavelength we can see (8). What makes a fluorescent object glow, however, is that the objects next to it do not have fluorescent properties. A fluorescent molecule takes a thermal or other non-visible source of energy and re-emits it at a different wavelength visible to the eye. Because a significant greater amount of light is being emitted by a fluorescent object than a non-fluorescent object, it appears to be glowing. The fluorescent materials are able to take non-visible energy and use it to emit more light. The non-fluorescent object can only emit the relatively small amount of visible light in the room (5). Harnessing non-visible energy, fluorescence is a way for an electron to emit light.
Uses of Fluorescence
History of Phosphorescence
Phosphorescence
Daily Uses of Phosphorescence
There are numerous uses for phosphorescence in daily life. It is used in the theater to mark ledges and stairs. When the lights go down for scene changes, the actors can still safely exit the stage. Apart from glow in the dark toys, phosphorescence is used for more sophisticated needs. Many watches are developed with phosphorescent materials on the hands so the user can tell the time in the dark. The kinds of phosphorus that are commonly found in nature display this property hence the name, phosphorescence (8).
Triboluminesence
Another way that atoms produce light is the process of triboluminescence. When you go into a very dark room and crunch on some mint Lifesavers, they create sparks in your mouth, but only if it is dry. This occurrence is fun and due to triboluminescence. When you crunch the Lifesavers, they tend to break along planes splitting positively and negatively charged ions. When one splits these sugar crystals, the ions try to bridge over the gap and create UV energy. This energy is absorbed by the candy, which is fluorescent, and re-emitted as visible light. This light is the spark one sees. Triboluminescence is just fluorescence in disguise (5).
Chemiluminescence
Chemiluminescence is a general process that consists of a reaction that produces molecules that become excited and release light. Some reactions that might have fluorescent or phosphorescent properties can also fall under the category of chemiluminescence. This property is very broad. All it involves is two molecules reacting to produce energy. This energy excites the product of the reaction. The excited molecule either releases a photon or transfers its energy to another molecule for emission. Bioluminescence is a subcategory of chemiluminescence. This process is how fireflies glow (7).
One of the earliest studies of chemiluminescence was by Henry Brand. This German alchemist was attempting to obtain gold from human urine. Instead, he obtained phosphorous that glowed in the air. This glowing was due to chemiluminescence. Luminol is a chemical that is used in devices to measure nitrogen dioxide in the air. Nitric oxide and ozone react together in a chemiluminescent reaction.
NO + O3 = NO2* + O2
NO2*= NO2 + hv
In this reaction, nitric dioxide is produced in an exited state denoted by the asterisk. This excited molecule emits a photon and completes the chemiluminescent reaction, which has about a ten percent yield. This reaction was used in the 1970’s for detecting harmful nitric oxide exhaust in cars. Putting dry air through electric discharge easily creates ozone. In these automobile tests, the sample exhaust and the ozone were mixed to create light. This light was then amplified then measured. The amplification allows for a broader range of samples of exhaust to be measured (7).
Glow sticks depend on chemiluminescence. The U.S. Navy first developed them for stealth missions. These sources of light are easily shielded if darkness is necessary. With this new technology, the U.S. Navy dropped behind enemy lines undercover. Divers in murky waters also use these light sticks extensively (7).
2H+ + C2O42- +H2O2 = 2CO2 + 2H2O
Hydrogen peroxide (H2O2) reacts with oxalate ester (C2O42- ) to produce two carbon dioxide (2CO2) molecules, water, and energy. This energy produced by the exothermic reactions is transferred to a fluorescent dye, which emits this energy as light. The fluorescence makes the stick “glow.” Another example of chemiluminescence at work is when metals glow blue when they get really hot. However, the yellow flame from burning wood is not chemiluminescence. Chemiluminescence is present in blacksmith’s shops all the way to the great depths of the ocean (7).
Introduction to Bioluminescence

The motion of the kayak is triggering the creatures in the water into lighting up and giving a bioluminescent show (10). But what is bioluminescence? Bioluminescence is the emission of light by organisms. This process is a sub-category of chemiluminescence. These creatures carry out a chemical reaction in their bodies. The intermediate products of this reaction are excited and then emit light (8). The Harbor Branch Oceanographic Institute found that between the depths of 200 to 1,000 meters, over ninety percent of organisms living there utilize bioluminescence (7). While fluorescence and phosphorescence just re-emit light at different wavelengths, bioluminescence produces the excited molecule without external energy as a factor. These three processes are comparable because their emission spectrums are similar. However, fluorescence and phosphorescence occurs in organisms too, but they are not the same as bioluminescence (4).
Big Picture Bioluminescence
Bioluminescence is a type of chemiluminescence that occurs in living organisms (4). Many organisms use this process for mating, schooling, camouflage, and hunting (8). These luminescent species lurk in marine habitats, in the air, and as terrestrial bacteria. Many marine sea creatures use bioluminescence. Some groups of animals, however, do not have any luminescent species. These groups include mammals, vertebras (not including fish), higher plants, and viruses. In labs, scientists have created luminescent versions of these animal groups by recombinant technology (4). Some scientists believe that the some species are able to be luminescent by eating other luminescent organisms (4).
On a larger scale, the reaction in bioluminescence is simple. A chemical substrate called luciferin, when catalyzed by luciferase, reacts with oxygen to produce oxyluciferin in an exited state. This product of the exothermic reaction is excited and releases a photon, or light! This reaction occurs in many sea creatures. Some of the key components are naturally produced in the organism or ingested. This basic reaction appears in variations across the many bioluminescent species (1).
Key Components in Bioluminescence
While studying firefly tales, scientists have discovers that there are a few key components in bioluminescent reactions that cannot be missing. Oxygen is one of the reactants that is needed to carry out a bioluminescent reaction (1). Fireflies breathe in oxygen though it’s complex systems of air tubes in order to deliver this ingredient to their stomachs containing luciferin (6). ATP (C10H16N5O13P3), or adenosine triphosphate, is essential to a bioluminescent reaction (11). It is fortunate that ATP is a key ingredient in a living organism. It is imperative that luciferin is present the reaction (1). The composition of luciferin in different creatures varies (1). This ingredient is oxidized by the enzyme luciferase. This enzyme kick starts the reaction by lowering the activation energy needed to start the reaction. From organism to organism, the enzyme luciferase can also vary. These ingredients are essential to making organisms glow by bioluminescence (4). In many types of species, luciferin must be brought in freshly for the reaction to occur. This process may happen by eating other luminescent creatures of producing luciferin within their body (1).
How Bioluminescent Reactions Occur

History of Bioluminescence
Luminescence has been observed for a long time, but it is only until very recently that we have been able to explain what iss happening. The ancient Greek and Chinese both recorded sighting of luminescence. Aristotle wrote about organisms releasing light over two thousand years ago! He said, “some things, though they are not in their nature fire, nor any species of fire, yet seem to produce light”(4). One phenomenon that scientists attribute to bioluminescence is milky seas. These very rare occurrences have been recorded by seamen for centuries. A milky sea is a rare occurrence where part of the Ocean emits a whitish blue light. These lights, which extend at times to forty miles, make the sea look milky. The first satellite image was taken January 25, 1995. This phenomenon still cannot be explained, but many scientists think that it is due to a high concentration of bioluminescent creatures. They compare it to a red tide, where algae accumulate in large bunches. In the novel Twenty Thousand Leagues Under the Sea the submarine passes through a milky sea. The parallels between an actual milky sea and the “sea of milk” described in the book make researchers conclude that this occurrence in the book was based on someone’s real account (1). Overall, the exact reason behind the occurrences of milky seas remains a mystery. Bioluminescence has been seen for centuries, but just only recently studied in depth.
Bioluminescence in Fireflies
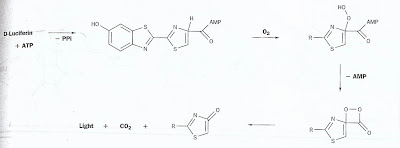
First, the D-luciferin and the adenosine triphosphate are present together. The D-luciferin uses up two of the adenosine triphosphate’s phosphates to form adenosine monophosphate. The new form of luciferin with AMP attached oxidizes with O2 gas to form a new compound. The AMP breaks away leaving another compound. Carbon dioxide (CO2) separates leaving an excited molecule that emits a photon (4) (9). The structure of luciferin in a firefly is C13H12N2O3S2 (8). A firefly stores both luciferin and luciferase in its sixth abdominal part. Oxygen comes through its air pipes and reacts the reaction starts when these ingredients are mixed (6). This reaction produces light that shines through fireflies’ stomachs. There are two different theories that scientist follow on the fireflies’ ability to flash their lights. Some believe that the fireflies regulate how much oxygen they intake, using oxygen as the limiting reagent in the reaction. If there is not enough oxygen, the light cannot be produced. Another theory is that fireflies have physical control over the production of light besides limiting the reactants. They think that fireflies use neural control to start the reaction (6). Fireflies use their bioluminescence for mating. It is remarkable that their ability to produce light requires little heat and is 96% efficient. The fireflies waste nine times less energy than incandescent light bulbs (6). Fireflies and beetles have a remarkable ability to produce light in an efficient manner.
Bioluminescence in Dinoflagellates

(4).
The luciferin in the structure of a tetrapyrrole in the presence of ATP is catalyzed by luciferase to react with O2. In this oxidation reaction, a new compound is formed. Water is a product that is removed leaving an excited molecule that emits a photon (4). The left over unexcited molecule is no longer useful and often disposed (1). These tiny but bioluminescent organisms are triggered to produce flashes of light by motion. The motion of waves or small organisms like fish is enough to trigger these dinoflagellates (1). Heat and pH affect the behavior of these species of algae. At pH 8 the luciferase is isolated from the luciferin prohibiting a reaction, while at a more acidic pH of 6, bioluminescence occurs (1). Although these dinoflagellates are small, when grouped together they can emit a lot of light.
Bioluminescence in Other Sea Creature
Uses of Bioluminescence
Conclusion
BIBLIOGRAPHY
(sorry the format is wacky)
(1) Haddock, Steven and James F. Case. “The Bioluminescence Web Page.” 30 Mar. 2009.
Biological Sciences at the University of California, Santa Barbara. 28 Apr. 2009
(2) Jennings, Paige. “ Glow with the Flow.” Scripps Institution of Oceanography. 28 Apr.
2009
(3) MacKenzie, Steven. "Bioluminescence." Gale Encyclopedia of Science. Ed. K. Lee
Lerner and Brenda Wilmoth Lerner. 4th ed. Detroit: Gale Group, 2008. Student Resource Center - Gold. Gale. CASTILLEJA HIGH SCHOOL BAISL. 28 Apr. 2009
(4) Meighen, Edward A.. “Bioluminescence.” Chemistry: Foundations and Applications.
2004 ed., 117-121.
(5) Rohrig, Brian. “A Light of a Different Color.” ChemMatters Apr. 1999 : 4-6.
(6) Shelton, Heather. “Bioluminescence: Fireflies and the Future.” 4 Jan. 2008. Serendip.
28 Apr. 2009
(7) Stedman, Donald H.. “Chemiluminescence.” Chemistry: Foundations and Applications.
2004 ed., 206-208.
(8) World of Chemistry. Detroit: Gale, 2000.
(9) McConnell, Jane. Helpful Discussion. 21 May 2009.
(10) “Bioluminescent Bay.” Nov. 20 2008. Island Adventures Biobay Tours 21 May 2009
(11) "Glow stick." Wikipedia, The Free Encyclopedia. 16 May 2009, 07:22 UTC. 22 May
2009
