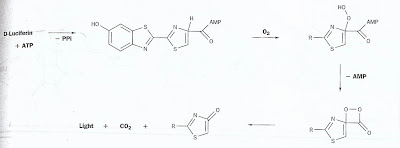
First, the D-luciferin and the adenosine triphosphate are present together. The D-luciferin uses up two of the adenosine triphosphate’s phosphates to form adenosine monophosphate. The new form of luciferin with AMP attached oxidizes with O2 gas to form a new compound. The AMP breaks away leaving another compound. Carbon dioxide (CO2) separates leaving an excited molecule that emits a photon (4) (9). The structure of luciferin in a firefly is C13H12N2O3S2 (8). A firefly stores both luciferin and luciferase in its sixth abdominal part. Oxygen comes through its air pipes and reacts the reaction starts when these ingredients are mixed (6). This reaction produces light that shines through fireflies’ stomachs. There are two different theories that scientist follow on the fireflies’ ability to flash their lights. Some believe that the fireflies regulate how much oxygen they intake, using oxygen as the limiting reagent in the reaction. If there is not enough oxygen, the light cannot be produced. Another theory is that fireflies have physical control over the production of light besides limiting the reactants. They think that fireflies use neural control to start the reaction (6). Fireflies use their bioluminescence for mating. It is remarkable that their ability to produce light requires little heat and is 96% efficient. The fireflies waste nine times less energy than incandescent light bulbs (6). Fireflies and beetles have a remarkable ability to produce light in an efficient manner.

No comments:
Post a Comment